Celltrion’s Remsima SC gains IBD indication approval in Europe
By Lim Jeong-yeoPublished : July 27, 2020 - 18:27
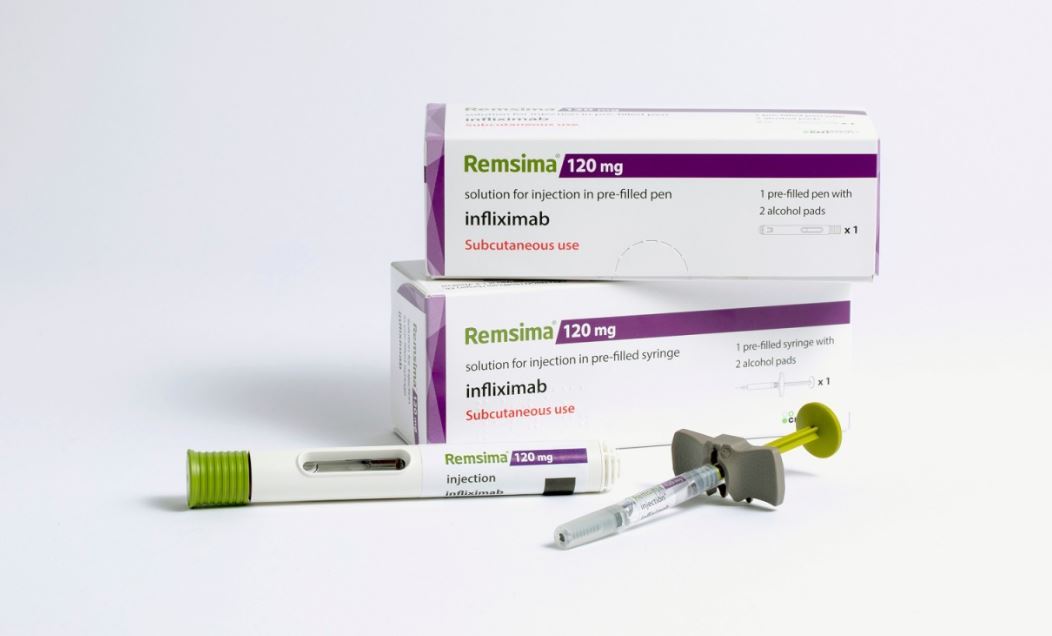
Remsima SC contends to be a biobetter, as the upgraded method of administration enables patients to self-administer the drug at home.
Amid the ongoing COVID-19 pandemic, which has disrupted conventional hospital drug dispensing systems, Remsima SC’s convenience is being seen in a new light.
Celltrion said the one-month period it took for the EMA to approve the drug for IBD since the Committee for Medicinal Products for Human Use recommended approval was an exceptionally fast time frame.
Remsima, an infliximab TNF-alpha autoimmune disease treatment, is also being clinically tested in the UK by scientists at Oxford University as a possible treatment for COVID-19 for its ability to contain an overreaction of the body‘s immune system called a “cytokine storm.”
With both rheumatoid arthritis and IBD treatment indications, Celltrion said that Remsima SC will serve as a vital instrument for the company’s strategy for the TNF-alpha inhibitor market, worth an estimated $46.8 billion as of 2019. The IBD market accounts for 30 percent of that, Celltrion said.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)







![[Graphic News] More Koreans say they plan long-distance trips this year](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050828_0.gif&u=)
![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)





![[From the Scene] Monks, Buddhists hail return of remains of Buddhas](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/19/20240419050617_0.jpg&u=20240419175937)

![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)

![[Today’s K-pop] Illit drops debut single remix](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/19/20240419050612_0.jpg&u=)