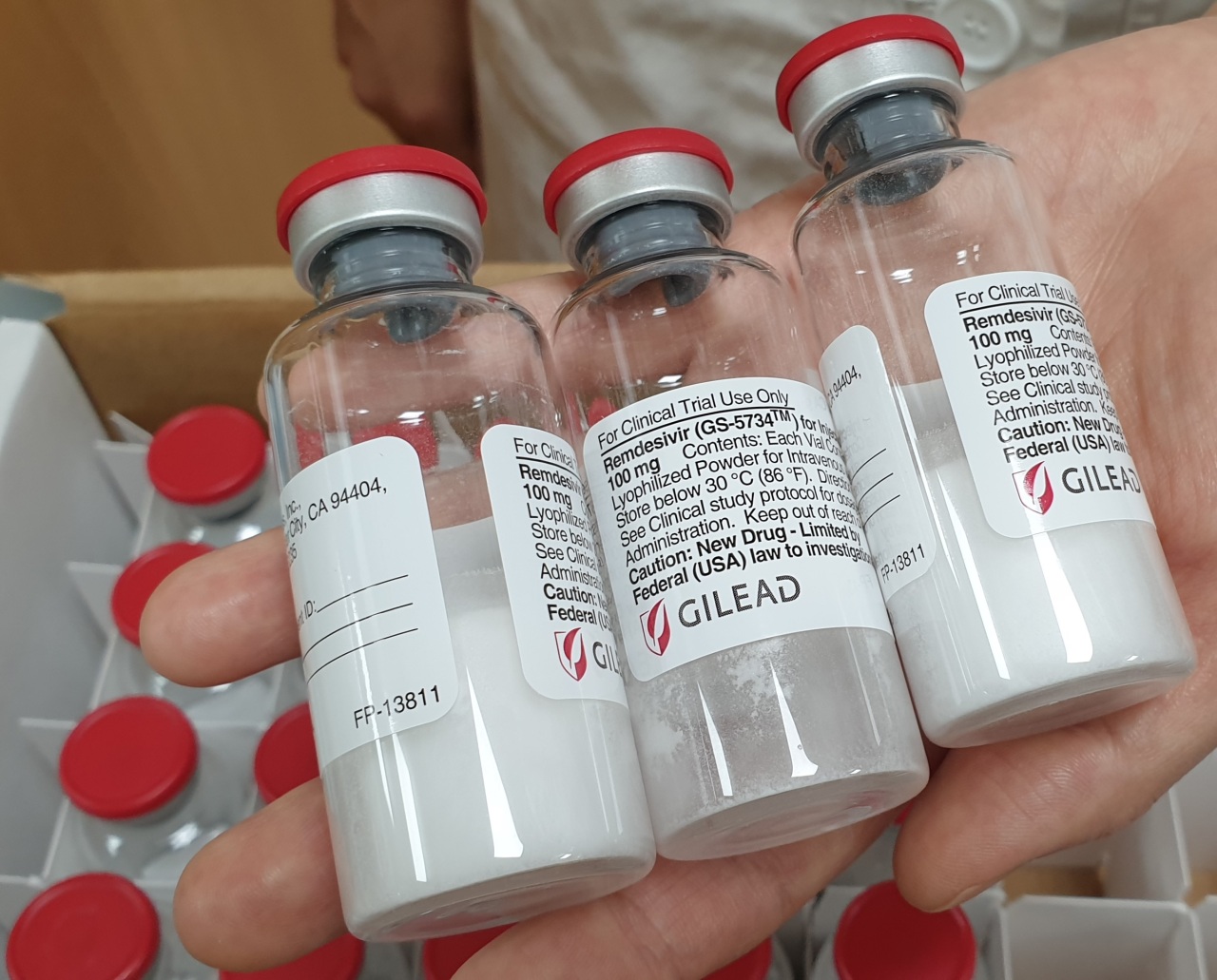
Korean health authorities said Wednesday that stocks of remdesivir, an experimental antiviral once seen as a potential Ebola therapy, will be supplied to local hospitals for treating COVID-19.
In a press release, the Korea Centers for Disease Control and Prevention said the use of remdesivir will be limited to seriously ill patients in need of oxygen therapy. More specifically, patients with chest scans showing signs of pneumonia and oxygen saturation of 94 percent or lower are eligible for the medication.
The stocks being distributed now have been donated by US pharmaceutical giant Gilead Sciences, the disease control agency said, without disclosing the exact amount of the doses. The agency said the donated supplies were projected to last for about a month. There are currently 33 patients with severe COVID-19 cases here, as of Wednesday.
The government said it plans to begin talks with a local importer to make purchases starting from August. The Food and Drug Safety Ministry granted approval for the import of remedesivir for emergency use last month. As Korea has yet to complete clinical tests on the drug, health authorities said they would be monitoring closely for possible side effects.
Gilead said the drug will be priced at $390 per vial, which roughly translates to $2,340 for the usual five-day course of treatment.
Remdesivir is so far the only effective therapy against COVID-19, although its proven benefits are modest. A preliminary report published May 22 on the New England Journal of Medicine showed the drug helped speed up the time to recovery by four days on average.
By Kim Arin (arin@heraldcorp.com)



![[Herald Interview] 'Amid aging population, Korea to invite more young professionals from overseas'](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/24/20240424050844_0.jpg&u=20240424200058)















![[Today’s K-pop] Kep1er to disband after 2 1/2 years: report](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/25/20240425050792_0.jpg&u=)