Animal worming tablets ineffective as cancer therapy: MFDS
Fenbendazole’s alleged cancer-curing claims quashed
By Lim Jeong-yeoPublished : Oct. 28, 2019 - 15:41
Using fenbendazole, a broad-spectrum drug used by veterinarians against gastrointestinal parasites --administered to sheep, cattle, horses, fish, dogs, cats, rabbits, and seals -- as treatment for cancer patients is inappropriate, the Korean Cancer Association and Ministry of Food and Drug Safety said Monday, citing lack of clinical data to vouch for its safety and efficacy.
Fenbendazole’s purported oncological effects became a global sensation after multiple reports from terminal cancer patients that they could partially recover. There have been many testimonies recently from cancer patients across the world endorsing its efficacy.
Despite the warnings of potential side effects from health authorities in Korea, some terminal cancer patients have broadcast their last-resort “self-clinical tests” on YouTube. Six tablets of fenbendazole can be purchased online for 42,000 won ($36).
Fenbendazole’s purported oncological effects became a global sensation after multiple reports from terminal cancer patients that they could partially recover. There have been many testimonies recently from cancer patients across the world endorsing its efficacy.
Despite the warnings of potential side effects from health authorities in Korea, some terminal cancer patients have broadcast their last-resort “self-clinical tests” on YouTube. Six tablets of fenbendazole can be purchased online for 42,000 won ($36).
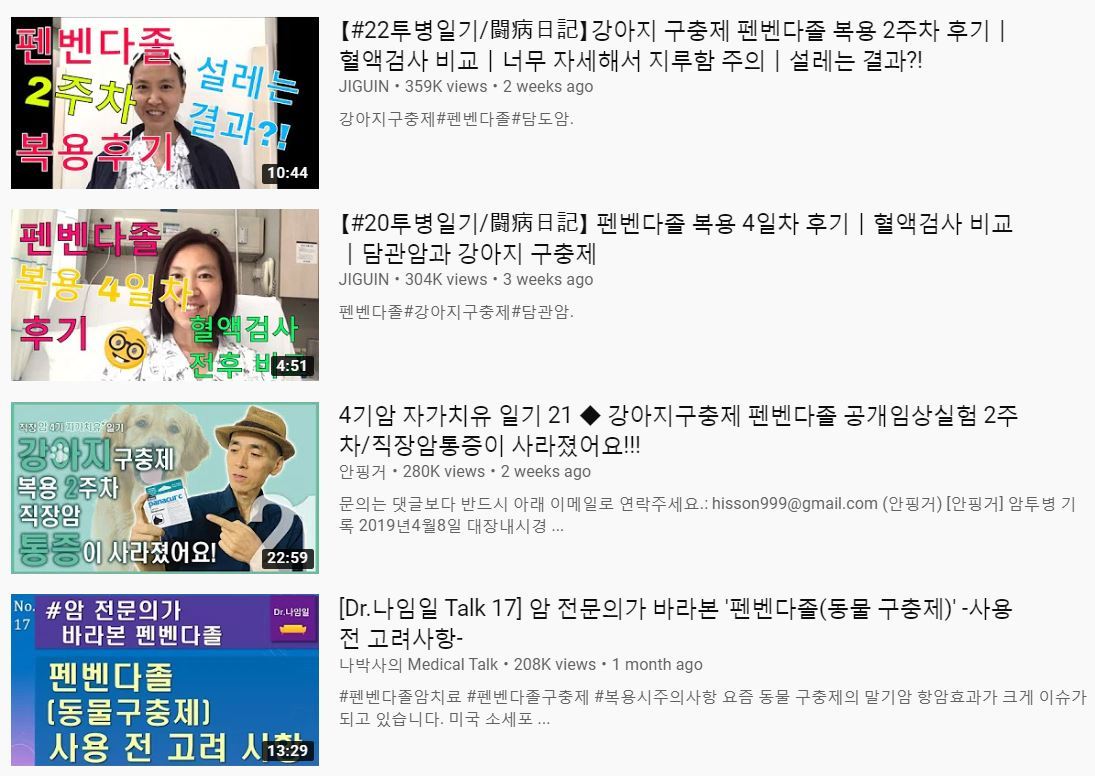
Korean oncological experts have dismissed the claims and termed it fake news.
“All drugs for cancer treatment must prove their safety and efficacy through human clinical trials,” according to medical experts.
Although there are scientific studies that point to the possibility of repurposing the veterinary drug as an anti-cancer agent, “the oncological effects of fenbendazole is based on experiments conducted on cells and animals, not humans,” the Korean authorities said.
The normal process for cancer treatment starts from the identification of novel drug candidates, followed by a cancer cell test and preclinical trials on animals. Then comes phase 1 clinical trials where a safe dosage for humans is researched, followed by the next stage where the drug’s effects on different types of cancer are tested. Finally, during the last phase of clinical trials the novel drug is compared with existing cancer drugs, before it is approved for actual sales.
There are existing cancer drugs actually approved for human use. These include, Vincristine which was approved in 1986, Vinblastine (1992), Vinorelbine (1995), Paclitaxel (1996) and Docetaxel (2006).
The Korea Cancer Association said with a low dosage for worming functions fenbendazole may show no side effects, but if the dosage increases for treating cancer, it may inflict serious damages to blood, nervous system and liver. A few fortuitous turnarounds cannot prove it is a viable cancer medicine, the KCA said.
The MFDS especially advised patients against taking a prescribed cancer medicine together with fenbendazole, citing the possibility of unexpected side effects, and said that it will continue to advise against its use by cancer patients.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)





![[From the Scene] Monks, Buddhists hail return of remains of Buddhas](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/19/20240419050617_0.jpg&u=20240419175937)





![[Graphic News] French bulldog most popular breed in US, Maltese most popular in Korea](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/18/20240418050864_0.gif&u=)


![[From the Scene] Monks, Buddhists hail return of remains of Buddhas](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/19/20240419050617_0.jpg&u=20240419175937)

![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)

![[Today’s K-pop] Illit drops debut single remix](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/19/20240419050612_0.jpg&u=)