First US FDA approval of CAR-T therapy hikes expectations for Korean biotech ventures
By Sohn Ji-youngPublished : Sept. 10, 2017 - 15:12
The US Food and Drug Administration’s first approval of a CAR-T cell therapy is raising market expectations for South Korean biotech ventures that are currently working to develop similar cellular immunotherapy drugs for cancer.
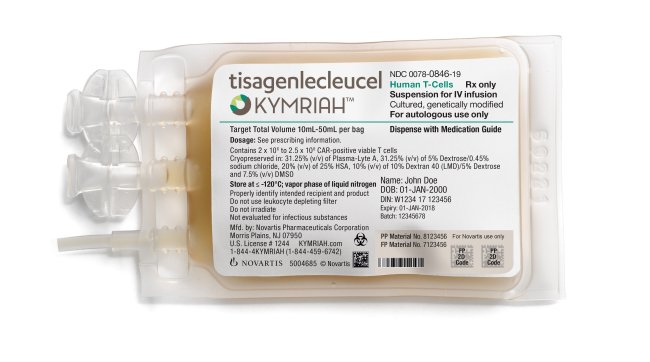
On Aug. 30, the US drug regulator approved Novartis’ Kymriah, a CAR-T cell therapy that genetically alters a patient’s own immune cells into a “living drug” that is trained to recognize and attack cancer cells in the blood.
It is the first CAR-T -- short for chimeric antigen receptor T-cell, the scientific name of the genetically modified white cells -- therapy to score approval internationally.
Kymriah, priced at a whopping $475,000, has been approved for children and young adults with B-cell acute lymphoblastic leukemia, an aggressive form of leukemia, which is refractory or has relapsed at least twice.
On Aug. 30, the US drug regulator approved Novartis’ Kymriah, a CAR-T cell therapy that genetically alters a patient’s own immune cells into a “living drug” that is trained to recognize and attack cancer cells in the blood.
It is the first CAR-T -- short for chimeric antigen receptor T-cell, the scientific name of the genetically modified white cells -- therapy to score approval internationally.
Kymriah, priced at a whopping $475,000, has been approved for children and young adults with B-cell acute lymphoblastic leukemia, an aggressive form of leukemia, which is refractory or has relapsed at least twice.
“As a breakthrough immunocellular therapy for children and young adults who desperately need new options, Kymriah truly embodies our mission to discover new ways to improve patient outcomes and the way cancer is treated,” Bruno Strigini, CEO of Novartis Oncology, said in a statement.
CAR-T cell therapies are widely expected to form a new wave in the realm of cancer treatments, going beyond traditional methods including surgery, chemotherapy, radiation therapy and targeted therapies -- drugs that treat cancer by targeting the specific molecular changes seen primarily in those cancerous cells.
The new approval has heightened market expectations for other players -- such as Gilead Sciences which recently acquired Kite Pharma, a pioneer in CAR-T cell therapies -- in the race to develop new cancer-targeting immunocellular drugs.
In Korea, a handful of biotechnology ventures, most notably Kosdaq-listed ViroMed, AbClon and Greeen Cross Cell, are developing CAR-T platform technologies and CAR-T cell therapies, though all of them are still in the preclinical development stage.
ViroMed licensed out its proprietary CAR-T platform technology to US-based Bluebird Bio in 2015. Under the deal, ViroMed has received an upfront payment of $1 million and is subject to additional milestone payments totaling up to $48 million.
The FDA’s recent approval of Kymriah appears to have helped raise the market’s expectations for ViroMed, whose share price rose to an all-time high of 145,000 won ($128.10) Friday. The shares closed at 143,900 won Friday, marking a 38 percent jump from a closing price of 103,900 won three months ago on June 8.
In addition, AbClon, which is currently pursuing a Kosdaq listing, has been working with Seoul National University since 2015 to develop its own CAR-T cell therapies. The team says it has a unique CAR-T platform that minimizes the onset of cytokine release syndrome, a common and potentially fatal complication of T-cell antibody infusion drugs.
Meanwhile, Green Cross Cell is developing CAR-T cell therapies targeting a range of other conventional cancers caused by solid tumors. The firm’s drugs are in the preclinical stage as of now.
By Sohn Ji-young (jys@heraldcorp.com)










![[Today’s K-pop] BTS pop-up event to come to Seoul](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050734_0.jpg&u=)
![[Graphic News] More Koreans say they plan long-distance trips this year](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050828_0.gif&u=)





![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)

